A great variety of chemicals are added to coating formulations. The most important components of a coating are the pigment and the binder. This section will therefore consider only these two components under the assumption that a slightly deeper discourse on the major components of the coating will be more informative than a cursory introduction to the large variety of chemicals that are routinely included in coating formulations. The largest component by mass of a coating formulation is the pigment. This is usually a mixture of calcium carbonate and clay. Various varieties of calcium carbonates and clays are available.
Clay has a lower whiteness and its use results in a smooth surface with a higher gloss level and higher opacity
Calcium carbonates
Calcium carbonates are distinguished from one another by their particle size distributions. Particle size distributions are often measured using sedimentation methods based on Stokes law.
Stokes law rearranges to:
h = Distance settled
t = Time


Pictures of calcium carbonate and clay taken with a scanning electron microscope.
Thus the rate at which the particles in a pigment slurry settle gives information about the size of those particles. There are alternative methods for determining the particle size distribution: these include the analysis of images of the pigment taken under high magnification and the analysis of the manner in which a sample of the pigment scatters light.
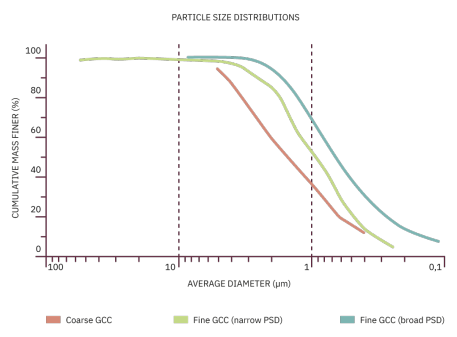
This chart shows the particle size distributions for three different calcium carbonates. The average diameter is a log scale, with the particle size becoming smaller towards the right. The gradient of this graph indicates the number of particles of a certain size. The steeper the gradient, the more particles of that size range there are in the pigment. The coarse GCC (ground calcium carbonate) has a gentle gradient compared to the other two samples shown. This indicates that the pigment consists of a broad range of particle sizes. The fine GCC with a broad particle size distribution has a similar gradient to the coarse GCC line but the gradient is shifted to significantly smaller particle sizes.
The fine GCC with a narrow particle size distribution has a steeper gradient than the other lines. This indicates that the particles of this pigment are of more uniform size than the particles of the other pigments shown.
Most of the calcium carbonates used in paper coatings are produced by grinding up a suitable mineral deposit and then filtering to produce the requisite particle size distribution. Calcium carbonate pigments can also be produced by deposition: the result of this process is a pigment with a relatively narrow distribution of particle sizes.
To understand the significance of the particle size distribution, imagine a mixture of three types of balls: footballs, handballs and tennis balls, which represent large, medium-sized and fine particles respectively. Imagine filling a box with a mixture of these balls to represent the coating process. When imagining the box, visualise three factors: the variation in the surface produced, the size and number of the openings in the surface and the amount
of free space in the packing. The first of these factors is obviously surface smoothness. The other two factors are important because narrow pores absorb ink vehicles more rapidly than large pores do. This may seem illogical, but in fact, the capillary pressure which drives the absorption is inversely proportional to the pore radius. The pore volume available also has an influence, if the pores are full then no further absorption can take place.
Exampels
|
|
Figure 1 |
|
|
Figure 2 |
|
|
Figure 3 |
|
|
Figure 1 |
|
|
Figure 2 |
|
|
Figure 3 |
Summary
This simple thought experiment illustrates the fact that particle size distribution is a key important factor which influences surface smoothness and the interaction between the paperboard and the ink during the printing process.
However, keep in mind that a great many other important factors also influence the performance of pigments in a real-life situation.
Clays
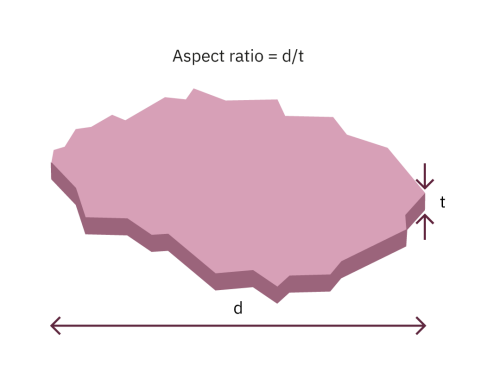
Clays are distinguished both by their particle size distribution and also by a property called the aspect ratio or shape factor.
A high aspect ratio indicates a very platy clay (a platy clay particle is one which is thin and wide like a plate as opposed to being thicker and narrower).
The aspect ratio varies depending on the size of the clay particles examined. Large clay particles tend to have high aspect ratios.
Smaller particles that have smaller aspect ratios tend to form surfaces that absorb ink rapidly compared to surfaces formed with a clay that has a high aspect ratio. Fast ink absorption requires a large number of narrow pores in the surface. It is easy to understand that a very platy clay will form a surface that has fewer pores than a surface formed with a clay that is less platy.
The ratio of clay to calcium carbonate used in the coatings depends on a number of factors. Clay is a very yellow pigment compared to calcium carbonate and has a significantly higher opacity. The higher opacity of the clay makes this pigment less compatible with OBAs (optical brightening agents) than calcium carbonate. Thus, if high whiteness is desired, there are advantages to having only a very small amount of clay in the coating. If high opacity is required then a higher clay content is advantageous.
Clay is a softer pigment than calcium carbonate. Clay has a Mohs ranking of 2.5 while calcium carbonate has a Mohs rating of 3.
Mohs scale
The Mohs scale is not a true scale but in fact an ordered list. The German mineralogist Friedrich Mohs took ten easily available minerals and arranged them in order of their ability to scratch one another. If a specimen can be scratched by a mineral on the list then it is softer than that mineral and has a lower Mohs rating, if it scratches the mineral then it has a higher Mohs rating than that mineral.
As clay is less abrasive than calcium carbonate, it causes less tool wear when the board is cut either during sheeting or converting operations. Coatings with a relatively high clay content therefore have an advantage in this respect. The clay content of a coating has a large impact on gloss, but this is a complex issue. When referring to gloss one must specify whether it is sheet gloss, print gloss or the difference between these two values (Snap) that is desirable. The clay content of the coatings is only one of a number of factors that affect gloss and it is beyond the scope of this discussion to consider this matter.
| Mohs Value | Mineral |
| 1 | Talc |
| 2 | Gypsum |
| 3 | Calcite |
| 4 | Flourspar |
| 5 | Apatite |
| 6 | Feldspar |
| 7 | Quartz |
| 8 | Topaz |
| 9 | Corundum |
| 10 | Diamond |
| Mohs Value | Mineral |
| 1 | Talc |
| 2 | Gypsum |
| 3 | Calcite |
| 4 | Flourspar |
| 5 | Apatite |
| 6 | Feldspar |
| 7 | Quartz |
| 8 | Topaz |
| 9 | Corundum |
| 10 | Diamond |
Latices
Latices is the plural form of the word latex. In the paper industry as a whole styrene-butadiene latices are the favoured binder. In circumstances where taint and odour considerations are important then it is more common to find styrene-acrylate binders.
The properties of latices can be varied by varying the proportion of the relevant monomers used, the molecular weight of the polymer molecules formed and the size of the latex particles formed.
Latices are often described by their glass transition temperature.
The glass transition temperature (Tg) is defined as follows: Tg refers to the temperature below which the polymer chains no longer have freedom of motion; above the Tg the polymer can be irreversibly deformed. In behavioural terms, below the Tg the latex is a hard, brittle substance, above the Tg the latex softens and its behaviour can be likened to that of chewing gum. A latex with a high Tg is often described as being hard while a latex with a low Tg is described as being soft. The properties of the latex used affect the properties of the coating layer formed.
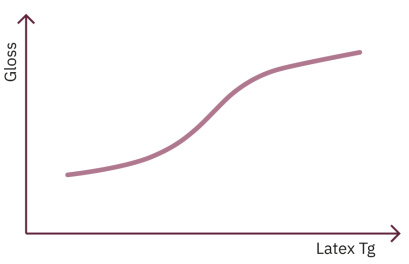
The top diagram illustrates how gloss can be affected by the glass transition temperature of the latex. The gloss levels reported in the diagram refer to uncalendered samples. Calendering is an operation whereby the board is pressed between a number of cylinders with the intention of smoothing the surface. This process usually increases sheet gloss. A coating layer formed with a soft latex (low Tg) is generally more easily deformed than a coating layer formed with a hard latex (high Tg) and so calendering tends to have more of an effect when a soft latex is used.
Other properties, most notably surface strength, are negatively affected by a high glass transition temperature. Surface strength is particularly important in offset printing, when a paperboard with a low surface strength is more likely to exhibit picking. Picking is a print defect that occurs when the paperboard surface is not sufficiently strong to maintain its integrity during the printing process and areas of the coating are ripped out of the surface leaving white spots in the printed area.
Water/mass retention
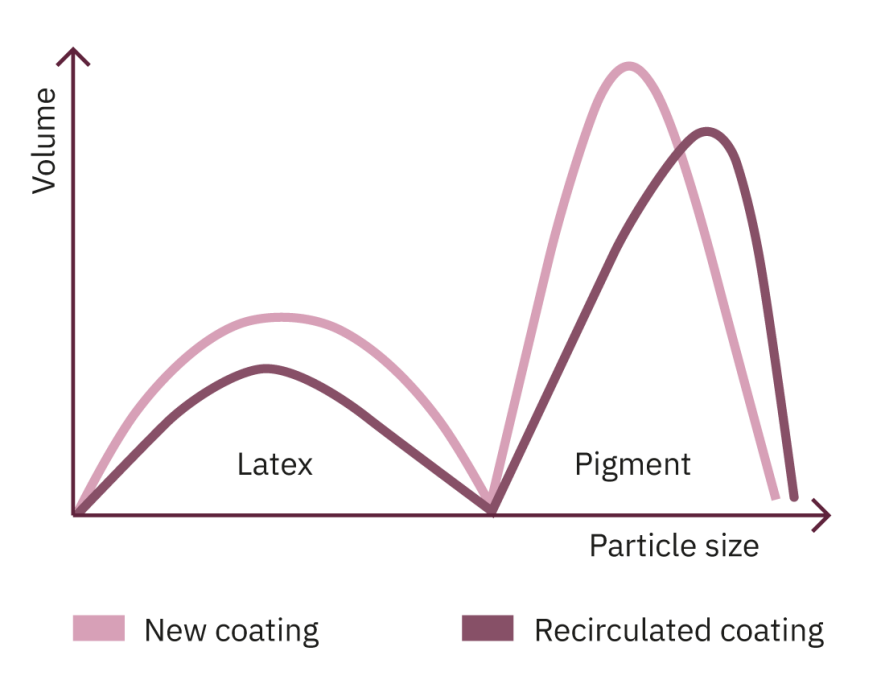
In the blade coaters the coating is applied to the baseboard using an applicator roll or a jet applicator. The sheet continues up to a blade that removes the excess coating. The excess coating is recirculated and reused. Because the coating is applied to a porous surface, some of the constituents of the coating formulation can be absorbed prior to a portion of the applied coating being removed by the coating blade. This coating is recirculated but is not quite the same as the fresh coating. As a result, over time the constant recirculation of the coating results in changes to the coating composition.
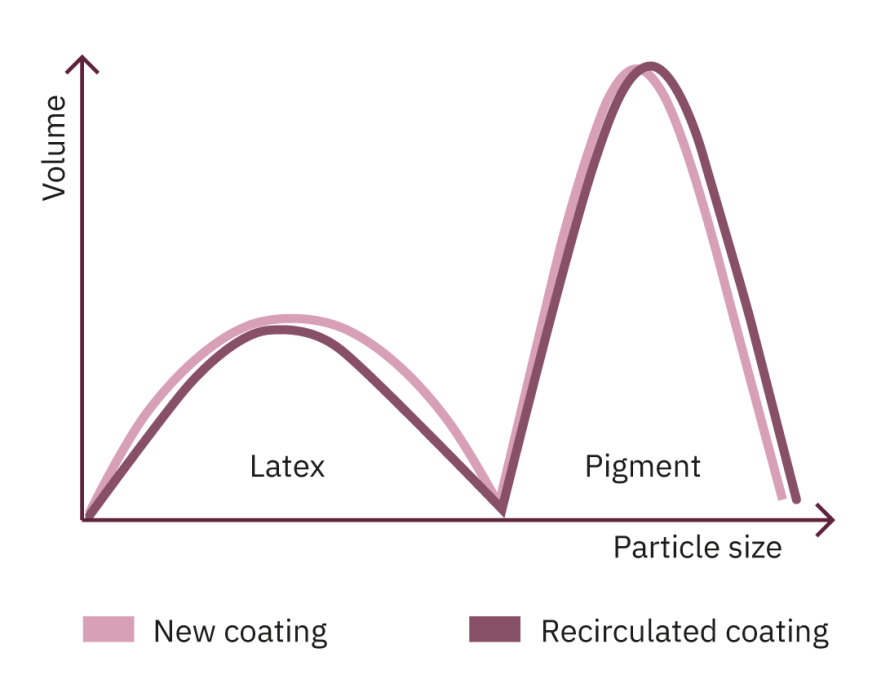
The above simplified diagrams are visual aids for understanding the changes that the coating undergoes during the coating process. The white lines represent the coating prior to use: the blue lines represent the coating after an extended period of time in the coating circulation systems. The graphs are bimodal: the peak at lower particle size is from the latex and the peak at higher particle size is from the calcium carbonate.
A representation of a coating containing both calcium carbonate and clay would have at least two pigment peaks, at least one for each pigment used.
The diagrams on the previous page illustrate the fact that during the production process, both latex and fine pigment particles are lost from the coatings. As a result, the proportion of large pigment particles increases and the pigment becomes increasingly coarse. The coating shown in the middle graph changes significantly less than the coating in the bottom graph. This means that there is a good chance that the sample in the middle will perform with greater consistency than the sample at the bottom. The sample in the middle has the better water/mass retention.
Summary
To summarise this section:
- Particle size distributions are often used to classify the pigments used in paper and board coatings.
- The particle size distribution of the specific pigments used affects the resulting paperboard properties such as smoothness and ink absorbency.
- The properties of the latex impacts on surface properties such as gloss and surface strength.
- The coating operation itself effects changes in the composition of the coating.